AP Chemistry – Class 58
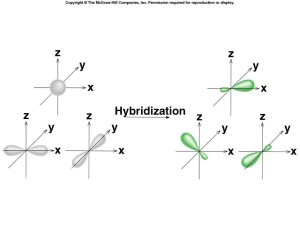
Hybridization
Dates
- Period E – Wednesday, January 23rd
- Period F – Wednesday, January 23rd
Big Idea: Where are the bonding electrons?
Limitations of atomic orbitals
- geometry of s, p, d, f orbitals?
- geometry of molecules?
- compare – do they match? no!
- need a different system
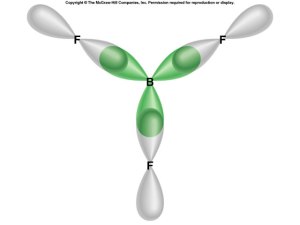
Bonding Orbitals
- explains location of electrons in a bonding situation
- think of as blending atomic orbitals
- perimeter atoms don’t hybridize
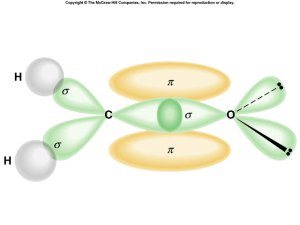
What about multiple bonds?
- must match geometry with hybridized orbitals
- count multiple as bonding area
- extra bonds use left over, unhybridized, “p” orbitals
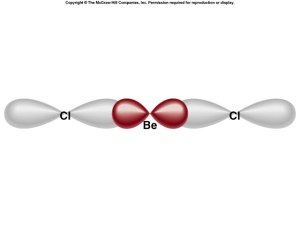
Overlap
- along axis = sigma
- parallel to axis = pi
Examples from Lab
Homework
- Assign hybridization to all atoms for the examples used during the lab.
Images Used During Class
sp3 Hybridization
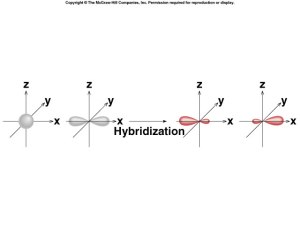
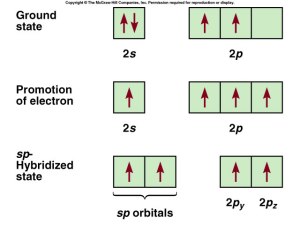
sp Hybridization
sp2 Hybridization
Sigma and Pi Bonding






You must be logged in to post a comment.